Abstract
Introduction: Ibrutinib, a once-daily Bruton's tyrosine kinase inhibitor (BTKi), is the only targeted therapy that has demonstrated overall survival benefits compared to chemo- and/or immunotherapy across multiple phase 3 clinical trials in first line (1L) and relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Evidence suggests that atrial fibrillation (AF) is a potential adverse event (AE) associated with all BTKi's used to treat patients with CLL/SLL. While CLL/SLL patients may have a high baseline risk of AF, little is known about the real-world (RW) outcomes of BTKi's such as ibrutinib for these patients. In this study, we describe time to next treatment (TTNT) and time to discontinuation (TTD) for CLL/SLL patients who initiated a 1L or second or later line (2L+) of therapy with ibrutinib or other regimens, in all patients and for subsets of patients with high baseline risk of AF/AF-related stroke.
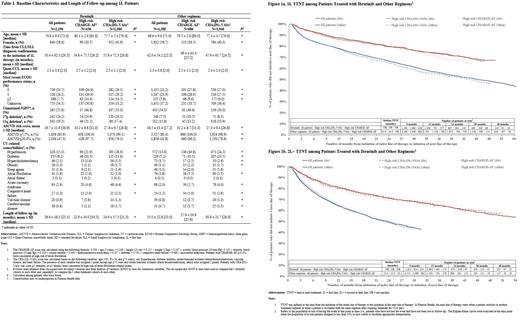
Methods: In this descriptive study, the nationwide Flatiron Electronic Health Record-derived de-identified database (02/12/2013-01/31/2021) was used to select patients with CLL/SLL who had ≥2 clinic visits and started 1L or 2L+ therapy with ibrutinib or other regimens (e.g., chemoimmunotherapy [CIT], other novel targeted agents) on or after 02/12/2014 (index date). For each setting (1L and 2L+) and index regimen (ibrutinib or other regimens), three cohorts were considered: all patients, patients at high risk of AF (CHARGE-AF risk score ≥10.0%), and patients at high risk of AF-related stroke (CHA 2DS 2-VASc risk score ≥3 [females] or ≥2 [males]). TTD was defined as the time from starting index therapy to the earliest of: (1) stopping treatment (when a gap of >120 days was observed after the last day of supply of continuous treatment), (2) the day before switching to a next line of therapy, or (3) the end of data availability if patients did not stop or switch treatment. TTNT was defined as the time from starting index therapy to the start of the next line of therapy. TTNT was compared between cohorts using Kaplan-Meier rates with log-rank P-values.
Results: A total of 2,190 (20% high risk CHARGE-AF [N=438], 49.3% high risk CHA 2DS 2-VASc [N=1,080]) and 1,851 (17.5% high risk CHARGE-AF [N=324], 44.5% high risk CHA 2DS 2-VASc [N=824]) patients were treated with ibrutinib in 1L and 2L+, respectively; while 4,388 (15.6% high risk CHARGE-AF [N=686], 44.3% high risk CHA 2DS 2-VASc [N=1,946]); and 4,135 (20.8% high risk CHARGE-AF [N=862], 50.5% high risk CHA 2DS 2-VASc [N=2,089]) patients were treated with other regimens in 1L and 2L+, respectively. Among other regimens, CIT was generally the most common regimen (42.9%-55.4% and 20.8%-26.7% of patients in 1L and 2L+, respectively). The prevalence of high risk del(17p) mutation at baseline was higher for patients treated with ibrutinib (24.0-25.5%) than for other regimens (7.5-14.2%). The mean Quan-Charlson comorbidity index score was ~2.3 and the prevalence of baseline cardiovascular comorbidities (e.g., hypertension, diabetes, and acute coronary syndrome), varied among cohorts in 1L and 2L+ (Table 1, 2L+ data not shown).
In the ibrutinib 1L cohorts, the mean follow-up length was 26.9 months in all patients, 22.9 months in high risk CHARGE-AF, and 24.9 months in high risk CHA 2DS 2-VASc, with mean TTD being 20.0, 16.1, and 18.0 months, respectively. Similar results were found in 2L+, for all patients and high risk cohorts.
TTNT was longer for all patients on ibrutinib (1L and 2L+ median TTNT not reached) than for all patients on other regimens (1L median TTNT: 45.9 months [Fig 1a]; 2L+ median TTNT: 23.6 months [Fig 1b], all P<0.05). Similar results were observed when comparing high risk patients on ibrutinib to high risk patients on other regimens (all P<0.05). For both ibrutinib and other regimens, there were no differences in TTNT between all patients and high risk cohorts, in 1L and 2L+ (all P>0.05; Fig 1a and 1b).
Conclusions: In this analysis, patients at a high risk for AF/AF-related stroke continued to demonstrate benefit with ibrutinib use across all lines of therapy. In addition, TTNT was significantly longer for patients treated with ibrutinib-based regimens than other regimens. Overall, this study confirms the RW benefits as demonstrated across multiple clinical trials and shows that baseline risk of AF/AF-related stroke does not adversely impact TTNT associated with ibrutinib use in patients with CLL/SLL.
Narezkina: Pharmacyclics, Janssen Scientific Affairs: Consultancy. Lu: Janssen Scientific Affairs: Current Employment; Johnson & Johnson: Current equity holder in publicly-traded company. Emond: Pharmacyclics LLC, an AbbVie Company: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Janssen: Consultancy; GlaxoSmithKline: Consultancy. Côté-Sergent: GlaxoSmithKline: Consultancy; Janssen: Consultancy. Hilts: Janssen: Consultancy; GlaxoSmithKline: Consultancy; ViiV: Consultancy; Allergan/Abbvie: Consultancy. Liu: Novartis: Consultancy; uniQure: Consultancy; Servier: Consultancy; Janssen: Consultancy; BioMarin: Consultancy. Lafeuille: GlaxoSmithKline: Consultancy; Janssen Scientific Affairs, LLC: Consultancy; Pfizer: Consultancy; Pharmacyclics: Consultancy. Lefebvre: Pharmacyclics: Consultancy; Otsuka: Consultancy; Novartis: Consultancy; Regeneron: Consultancy; Pfizer: Consultancy; Janssen Scientific Affairs, LLC: Consultancy; GlaxoSmithKline: Consultancy. Huang: Janssen Scientific Affairs, LLC: Current Employment; Johnson & Johnson: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal